Genes certainly play a significant role in the development of Alzheimer’s. Over the past few decades, lots of research has been conducted in order to establish how far genes influence the development of Alzheimer’s disease. Familial Alzheimer’s is a rare form of the disease where a combination of inherited mutations guarantees the development of the disease. People with Familial Alzheimer’s begin to have symptoms at a much earlier age, which is why Familial Alzheimer’s is also known as Early Onset Alzheimer’s.
The mutations involved with Alzheimer’s
With advances in technology, it has become easier for geneticists to identify the specific mutations that are associated with Alzheimer’s. Some of the most important genetic research for Alzheimer’s to date has been the identification of the mutations in the amyloid precursor protein (APP), Apolipoprotein E (APOE) and presenilin proteins (PSEN1 or PSEN2). APOE is strongly linked to the development of late-onset Alzheimer’s. Mutations in PSEN1, PSEN2 and APP are known to cause early-onset forms of Alzheimer’s but are also known to increase the risk for the late-onset form.
The mutations involved with Alzheimer’s
With advances in technology, it has become easier for geneticists to identify the specific mutations that are associated with Alzheimer’s. Some of the most important genetic research for Alzheimer’s to date has been the identification of the mutations in the amyloid precursor protein (APP), Apolipoprotein E (APOE) and presenilin proteins (PSEN1 or PSEN2). APOE is strongly linked to the development of late-onset Alzheimer’s. Mutations in PSEN1, PSEN2 and APP are known to cause early-onset forms of Alzheimer’s but are also known to increase the risk for the late-onset form.
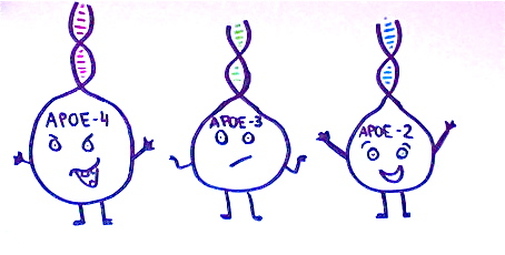
APOE occurs in three forms; APOE2, APOE3 and APOE4. Scientists believe the APOE4 allele encourages the formation of amyloid plaques, and it is known as the most significant risk factor for late-onset Alzheimer’s.
25% of the population inherits one copy of the APOE4 allele of the APOE gene, thus increasing the risk of Alzheimer’s by four times. 2% of the population inherit two copies of this allele, increasing their risk of Alzheimer’s by eight times. APOE codes for alipoprotein E which aids transportation of cholesterol to the body’s cells. Common mutations of this gene are also known to increase the risk of stroke and cardiovascular disease, which could explain why the conditions are linked (Lane, N. 2002 p290).
The other APOE alleles have different effects. 60% of people inherit two copies of the APOE3 allele, and are said to be at an ‘average risk’. It is believed that while APOE3 has somewhat neutral effects on the development of Alzheimer’s, APOE2 may have some protective effects. 11% of the population will inherit one copy of APOE2 and one copy of APOE3, but it is much more rare to inherit two copies of APOE2. Scientists believe that while these alleles do not prevent Alzheimer’s, they may delay the time of onset.
25% of the population inherits one copy of the APOE4 allele of the APOE gene, thus increasing the risk of Alzheimer’s by four times. 2% of the population inherit two copies of this allele, increasing their risk of Alzheimer’s by eight times. APOE codes for alipoprotein E which aids transportation of cholesterol to the body’s cells. Common mutations of this gene are also known to increase the risk of stroke and cardiovascular disease, which could explain why the conditions are linked (Lane, N. 2002 p290).
The other APOE alleles have different effects. 60% of people inherit two copies of the APOE3 allele, and are said to be at an ‘average risk’. It is believed that while APOE3 has somewhat neutral effects on the development of Alzheimer’s, APOE2 may have some protective effects. 11% of the population will inherit one copy of APOE2 and one copy of APOE3, but it is much more rare to inherit two copies of APOE2. Scientists believe that while these alleles do not prevent Alzheimer’s, they may delay the time of onset.
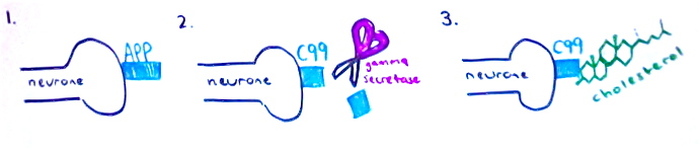
Mutations in PSEN1, PSEN2 and APP are known to cause early-onset forms of Alzheimer’s by resulting in the overproduction of beta amyloid.
Presenilin proteins are known to affect the enzyme gamma secretase. This is one of the enzymes which acts on the APP protein to form amyloid beta fragments. Inheritors of the two variants PSEN1 and PSEN2 are guaranteed to develop early-onset Alzheimer’s However studies have now shown evidence to suggest that carrying either of these mutations also increases the risk of developing the late-onset form.
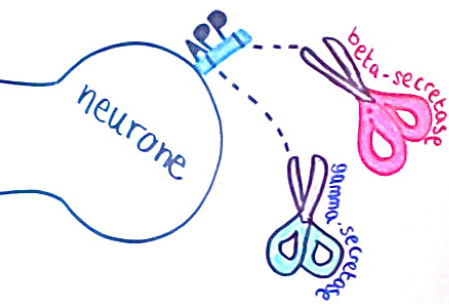
Amyloid beta precursor protein (APP) is a cell surface receptor in neurones. It is the substrate of beta-secretase and gamma-secretase, which ‘cut’ the protein to produce amyloid beta. APP is first cut by beta-secretase to produce the protein C99. Recent studies from scientists at Vanderbilt University have suggested that C99 needs to bind to cholesterol to be cut by gamma-secretase. Once the C99 has bound to the cholesterol molecule it moves to ‘lipid rafts’ in the cell membrane where it is cut to form amyloid beta. This could explain why high levels of cholesterol influence the development of Alzheimer’s disease.
Recently, scientists identified a mutation in APP that seemed to have preventative effects. They sequenced 1,795 Icelanders and found that 0.5% had an APP mutation of the allele A673T. This allele seemed to give them a 50% greater chance of reaching 85 without developing Alzheimer’s. The study provides evidence of the influence of genetics on the development of Alzheimer’s.
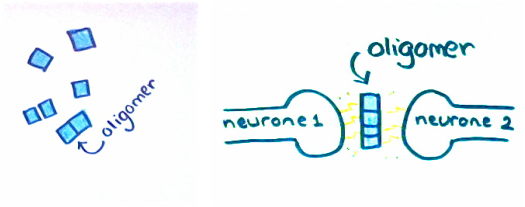
The genetic causes result directly in the overproduction of amyloid beta. If it can be proven that the protein plaques are important to the development of the disease, new drug targets can be made. These gene mutations are known as ‘susceptibility genes’. Each gene influences the development of Alzheimer’s to a small degree. However, the larger the number of susceptibility genes a person has, the greater the risk of Alzheimer’s development. Genome-wide association studies are now being carried out to identify more of these susceptibility genes.
Got any comments or questions? Please feel free to post them!
* UPDATE
A new susceptibility gene for Alzheimer's has recently been identified. Certain variants of the TREM2 gene are thought to be linked to late onset Alzheimer's following a study involving Icelandic subjects. The TREM2
gene is involved in activating an immune response within the CNS, where it has an anti-inflammatory role. A mutation in this gene would result in a decreased ability of the brain to clear away amyloid plaques, and an overactive inflammatory response.
See the article in Nature; Nature Reviews Neurology 9, 5 (January 2013)
http://www.nature.com/nrneurol/journal/v9/n1/full/nrneurol.2012.254.html
References
Alzheimer’s Association, 2012. Alzheimer’s Disease Facts and Figures, Alzheimer’s & Dementia, [pdf] Volume 8, Issue 2. Available at: <http://www.alz.org/downloads/facts_figures_2012.pdf> [Accessed 12 July 2012]
Cruchaga C, Chakraverty S, Mayo K, Vallania FLM, Mitra RD, et al.,2012 Rare Variants in APP, PSEN1 and PSEN2 Increase Risk for AD in Late-Onset Alzheimer's Disease Families, PLoS ONE, 7(2) [online] Available at: <http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0031039> [Accessed 13 July 2012]
HBO : Documentaries : The Alzheimer’s Project, 2012. The Connection between Insulin and Alzheimer’s [online video] Available at: <http://www.hbo.com/alzheimers/supplementary-the-connection-between-insulin-and-alzheimers.html> [Accessed 12 July 2012]
Jonsson, T. et al, 2012. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline, Nature [online] Available at: <http://www.nature.com/nature/journal/vaop/ncurrent/full/nature11283.html> [Accessed 18 July 2012]
Lane, N., 2002. Oxygen: The Molecule that made the World. Great Britain: Oxford University Press
NCBI, 2012. PSEN1, Presenilin 1 [Homo Sapiens [online Gene ID] Available at: <http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=5663#bibliography> [Accessed 18 July 2012]
MacMillan, L. 2012. Alzheimer’s protein structure suggests new treatment directions, Research News at Vanderbilt [online] Available at <http://news.vanderbilt.edu/2012/05/alzheimers-protein-structure/> [Accessed 14 July 2012]
Presenilin proteins are known to affect the enzyme gamma secretase. This is one of the enzymes which acts on the APP protein to form amyloid beta fragments. Inheritors of the two variants PSEN1 and PSEN2 are guaranteed to develop early-onset Alzheimer’s However studies have now shown evidence to suggest that carrying either of these mutations also increases the risk of developing the late-onset form.
Amyloid beta precursor protein (APP) is a cell surface receptor in neurones. It is the substrate of beta-secretase and gamma-secretase, which ‘cut’ the protein to produce amyloid beta. APP is first cut by beta-secretase to produce the protein C99. Recent studies from scientists at Vanderbilt University have suggested that C99 needs to bind to cholesterol to be cut by gamma-secretase. Once the C99 has bound to the cholesterol molecule it moves to ‘lipid rafts’ in the cell membrane where it is cut to form amyloid beta. This could explain why high levels of cholesterol influence the development of Alzheimer’s disease.
Recently, scientists identified a mutation in APP that seemed to have preventative effects. They sequenced 1,795 Icelanders and found that 0.5% had an APP mutation of the allele A673T. This allele seemed to give them a 50% greater chance of reaching 85 without developing Alzheimer’s. The study provides evidence of the influence of genetics on the development of Alzheimer’s.
The genetic causes result directly in the overproduction of amyloid beta. If it can be proven that the protein plaques are important to the development of the disease, new drug targets can be made. These gene mutations are known as ‘susceptibility genes’. Each gene influences the development of Alzheimer’s to a small degree. However, the larger the number of susceptibility genes a person has, the greater the risk of Alzheimer’s development. Genome-wide association studies are now being carried out to identify more of these susceptibility genes.
Got any comments or questions? Please feel free to post them!
* UPDATE
A new susceptibility gene for Alzheimer's has recently been identified. Certain variants of the TREM2 gene are thought to be linked to late onset Alzheimer's following a study involving Icelandic subjects. The TREM2
gene is involved in activating an immune response within the CNS, where it has an anti-inflammatory role. A mutation in this gene would result in a decreased ability of the brain to clear away amyloid plaques, and an overactive inflammatory response.
See the article in Nature; Nature Reviews Neurology 9, 5 (January 2013)
http://www.nature.com/nrneurol/journal/v9/n1/full/nrneurol.2012.254.html
References
Alzheimer’s Association, 2012. Alzheimer’s Disease Facts and Figures, Alzheimer’s & Dementia, [pdf] Volume 8, Issue 2. Available at: <http://www.alz.org/downloads/facts_figures_2012.pdf> [Accessed 12 July 2012]
Cruchaga C, Chakraverty S, Mayo K, Vallania FLM, Mitra RD, et al.,2012 Rare Variants in APP, PSEN1 and PSEN2 Increase Risk for AD in Late-Onset Alzheimer's Disease Families, PLoS ONE, 7(2) [online] Available at: <http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0031039> [Accessed 13 July 2012]
HBO : Documentaries : The Alzheimer’s Project, 2012. The Connection between Insulin and Alzheimer’s [online video] Available at: <http://www.hbo.com/alzheimers/supplementary-the-connection-between-insulin-and-alzheimers.html> [Accessed 12 July 2012]
Jonsson, T. et al, 2012. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline, Nature [online] Available at: <http://www.nature.com/nature/journal/vaop/ncurrent/full/nature11283.html> [Accessed 18 July 2012]
Lane, N., 2002. Oxygen: The Molecule that made the World. Great Britain: Oxford University Press
NCBI, 2012. PSEN1, Presenilin 1 [Homo Sapiens [online Gene ID] Available at: <http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=5663#bibliography> [Accessed 18 July 2012]
MacMillan, L. 2012. Alzheimer’s protein structure suggests new treatment directions, Research News at Vanderbilt [online] Available at <http://news.vanderbilt.edu/2012/05/alzheimers-protein-structure/> [Accessed 14 July 2012]

 RSS Feed
RSS Feed